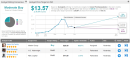
The old cliché of “a game of two halves” can apply almost perfectly to Inovio Pharmaceuticals’ (INO) 2020. Until roughly the end of June, shares of the formerly little-known biotech were on an absolute tear. As one of the smaller companies to benefit from the market’s infatuation with “coronavirus stocks,” the company’s valuation soared on the premise of the successful development of INO-4800, its DNA COVID-19 vaccine candidate.But since then, sentiment has soured as the vaccine’s development has not gone smoothly. Progress hit a brick wall in September, when the FDA halted the planned Phase 2/3 study of INO-4800. The regulatory body cited the need for more information on INO-4800 and the company’s CELLECTRA 2000 delivery device.However, Inovio reported Q3 results this week and the market has reacted favorably, sending shares up by 35% in Tuesday’s trading session. Inovio, though, is still developing its pipeline of vaccines and has no product to sell, and the renewed optimism has nothing to do with the quarterly numbers but more to do with the prospect of the company re-entering the Covid-19 vaccine race. Inovio disclosed that, as required, it has responded to the FDA’s questions regarding INO-4800 and anticipates an FDA response later this month.This latest development is encouraging, says H.C. Wainwright analyst Ram Selvaraju. Whilst the successful trial data from Pfizer/BioNtech’s offering means a vaccine is likely to hit the market soon, that does not exclude Inovio joining the fray with its differentiated offering further down the line.“We estimate that the trial could start by the end of 2020 and enroll patients in a timely fashion as COVID-19 infections are undergoing a massive resurgence across the U.S. We estimate that this Phase 2/3 trial could report data in 2H21. We believe the first approved COVID-19 vaccine may enter the market in early 2021, but that does not obviate the need to develop other COVID-19 vaccine candidates. mRNA-based vaccines need frozen cold chain logistics (storage at -94ºF or -70ºC), which is not necessary for DNA-based vaccines such as Inovio’s INO-4800,” the 5-star analyst said.Until more clarity arises, Selvaraju remains on the sidelines with a Neutral (i.e. Hold) rating on INO shares. (To watch Selvaraju’s track record, click here)Inovio gets mostly Holds from Selvaraju’s colleagues, too – 5, as it happens. Boosted by an additional 2 Buys, the analyst consensus rates the stock a Moderate Buy. Given the $13.57 average price target, the Street’s forecast is for 18% upside over the coming months. (See INO stock analysis on TipRanks)To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.Disclaimer: The opinions expressed in this article are solely those of the featured analyst. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.,
,
