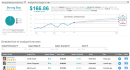
Johnson & Johnson said that Phase1/2 trial data showed that a single dose of its COVID-19 vaccine candidate, JNJ-78436735, induced a “strong” neutralizing antibody response in nearly all participants aged 18 years and older.Interim analysis from the ongoing Phase 1/2a clinical trial demonstrated that J&J’s (JNJ) vaccine candidate was generally well-tolerated. Immune responses were similar across the age groups studied, including older adults. J&J noted that the data are consistent with preclinical studies,which showed that a single dose of the vaccine prevented subsequent infection and provided complete protection in the lungs of non-human primates.The development of detectable antibodies was found in 99% of participants aged 18-55 years of age in the Phase 1/2 trial. The data showed that 98% of participants were positive for neutralizing antibodies against SARS-CoV-2 at day 29 post-vaccination. Immunogenicity data from participants aged 65 years of age and above were available for the first 15 participants, with strong humoral and cellular immune responses elicited in all elderly participants who received a single dose of the COVID-19 vaccine candidate.The ongoing Phase 1/2a clinical trial aims to test the safety and immunogenicity of two dose levels of the COVID-19 vaccine, and as single and two-dose schedules. Based on the interim findings, J&J on Wednesday kicked off a large-scale, pivotal, multi-country Phase 3 trial of its COVID-19 vaccine candidate, which seeks to enroll up to 60,000 volunteers across three continents, the company added.The company also plans to run a Phase 3 clinical trial of a two-dose regimen of JNJ-78436735 versus placebo later this year.JNJ shares have almost fully recovered since plunging to a multi-year low in March and are now trading 0.1% lower than at the start of year. (See JNJ stock analysis on TipRanks).Credit Suisse analyst Matt Miksic last week reiterated a Buy rating on the stock with a $163 price target (12% upside potential), saying that JNJ’s Covid-19 vaccine candidate remains on track for emergency use approval (EUA) in early 2021.“We continue to view the program as more of a qualitative positive to the stock and valuation in the near-term, despite the $10+ billion annual sales potential in 2021 and 2022,” Miksic wrote in a note to investors.Overall, the rest of the Street shares Miksic’s bullish outlook on the stock. The Strong Buy analyst consensus boasts 7 unanimous Buy ratings. That’s with a $166.86 average price target indicating upside potential of 15% in the coming 12 months.Related News: J&J Kicks Off Phase 3 Covid-19 Vaccine Trial With Single Dose RedHill Gets Brazil’s Nod For Covid-19 Study With Opaganib Teva Launches Two Digital Inhalers For Asthma Patients More recent articles from Smarter Analyst: * Lockheed Ramps Up Dividend By 8.3%, Boosts Share Buyback By $1.3B * Delta To Incur Up To $2.5B In Jet Retirement Charges In 3Q * Vertex or Galapagos: Which Biotech Stock Does The Street Favor? * Raytheon Wins $212.8M US Missile Contract For UAE; Street Is Bullish,
,
