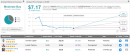
11 months ago, the U.S. Food and Drug Administration (FDA) shut down Solid Biosciences’ (SLDB) “IGNITE DMD” Phase I/II clinical trial after one of the young patients involved in the trial suffered a “serious adverse event.” Despite the patient in question recovering, no new patients suffering such serious adverse events, and the company providing the FDA with “information and measures intended to improve patient safety” and “data related to manufacturing process improvements,” the FDA declined to allow trials to resume in July, and asked Solid Biosciences to provide even more information on its trials, and on the safety measures it proposed to add to get the trials back on track.Fast forward three more months and — success!On Thursday, Solid Biosciences confirmed that the FDA has “lifted the clinical hold” on its trials, acknowledging that the company has “satisfactorily addressed all clinical hold questions.” Going forward, the company plans to remove most empty viral capsids (the protein shell that contains viral material) and limit the maximum weight of the child-patients entering its trials to 18 kilograms, so as to reduce the viral load of the SGT-001 therapy to which patients are exposed. As the name suggests, Solid Biosciences’ IGNITE DMD trials are aimed at slowing or reversing the effects of Duchenne Muscular Dystrophy (DMD), a genetic disease that causes progressive muscle degeneration and weakness mostly in young boys aged two and up (which explains the low maximum weight of trial participants). The company’s SGT-001 is a gene therapy that injects a modified virus into a patient, containing DNA coded to fix the problems that cause the disease.At least, this is what should happen in theory. The IGNITE DMD trials are designed to find out if it works in practice, and thanks to the FDA’s reconsideration, now these trials will be allowed to resume — probably in the first quarter of 2021.In a note Thursday, Chardan analyst Gbola Amusa confirmed that the FDA’s decision puts development of SGT-001 “back on course” towards addressing a $50 billion global market opportunity. If approved, the treatment will be one of only a “relatively few” gene therapies that have ever been approved for human use globally, putting Solid Biosciences in a leading position in this market. And because gene therapy products such as SGT-001 are considered “novel, complex, and relatively difficult to manufacture,” the barriers to entry in this market for competitors would be significant.The analyst views this as a “vast opportunity” — one not without risks, but one that could, if the treatment proves out, lift Solid Biosciences stock from its present price of $4 and change all the way to $12.50 or more. This new price target, by the way, is more than twice what Amusa valued the company at before the FDA’s Thursday decision, and implies 208% upside from current levels. And in fact, Amusa muses that “with progress,” the treatment “could take SLDB significantly past our updated price target of $12.50” — and notes repeatedly that in years past, Solid Biosciences stock even reached “peak highs of >$50/sh,” implying that the eventual upside could be even greater than what he’s prepared to postulate today. (To watch Amusa’s track record, click here)Overall, SLDB holds a Moderate Buy rating from the analyst consensus, based on 2 “buy,” and 1 “hold” ratings. Shares are selling for $4.06, and the average price target of $7.17 implies a 79% upside potential. (See SLDB stock analysis on TipRanks)To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.Disclaimer: The opinions expressed in this article are solely those of the featured analysts. The content is intended to be used for informational purposes only. It is very important to do your own analysis before making any investment.,
,
